CASCADE-Trial

Same-day ART initiation versus clinic-based pre-ART assessment and counselling for individuals newly tested HIV-positive during community-based HIV testing in rural Lesotho – a randomized controlled trial (CASCADE trial)
Background
In November 2014 the Joint United Nations Programme on HIV/AIDS (UNAIDS) published the 90-90-90 targets for 2020. The targets are that 90% of HIV-infected individuals know their HIV-status, of these 90% receive sustained anti-retroviral therapy (ART) and 90% of these achieve viral suppression. If achieved, the 90-90-90 targets will result in 73% of all HIV-infected persons having a suppressed viral load (VL) by 2020. The UNAIDS estimate that achievement of the 90-90-90 goal would result in a reduction of new HIV-infections from 2 million at present to 500'000 per year. The rationale of the 90-90-90 targets lies in the preventive potential of ART. Several studies have provided strong evidence that HIV infected individuals who achieve viral suppression through ART are virtually non-infectious to their sexual partners, thus reducing the risk of further transmission of HIV.
The 90-90-90 targets imply a "seek-test-treat" strategy aiming at diagnosing HIV-infections as early as possible with subsequent fast-track initiation of ART. Such a strategy bears, however, unprecedented challenges in settings where HIV is hyperendemic and resources may be limited.
 Going from door to door for HIV testing in one of the villages in Butha-Buthe District
Going from door to door for HIV testing in one of the villages in Butha-Buthe District
The Continuum of Care Cascade ("the cascade") involves the steps HIV-infected individuals have to take in order to achieve viral suppression. It starts with knowing one's HIV status, continues with linkage to care after a positive HIV test, initiation of ART, uninterrupted continuation of ART (retention in care and adherence to medication), and ends with viral suppression. Already prior to announcement of the "seek-test-and-treat" approach, weaknesses in the cascade often hampered the effectiveness of HIV programs in resource-rich as well as resource-poor settings. In Sub-Saharan Africa the care cascade is still far from the 90-90-90 targets with only 29% of infected individuals estimated to be on ART and virally suppressed in 2013. In order to achieve the UNAIDS targets innovative, effective, and practical approaches for improving the care cascade are thus urgently needed.
Rationale for the CASCADE trial
Several observational studies imply that for individuals who test HIV positive, starting antiretroviral therapy is often a protracted process. Individuals have to travel several times to their clinic to do the so called pre-ART assessment and pre-ART counseling. Many persons get discouraged by these lengthy procedures that imply transport cost to health care facilities and long waiting times at the clinics. Studies report that high shares of patients who were initially motivated to start ART are discouraged and jump off before they even started antiretroviral therapy.
The two arms in CASCADE trial
Individuals who test HIV-positive during home-based HIV testing are randomized in one of two arms. The control arm follows standard of care. Standard of care implies referral to the nearest clinic. The individual has then to attend the clinic for conduction of baseline laboratory tests and preparatory pre-ART adherence counselling. In the intervention group individuals receive baseline laboratory tests as point-of-care at their homes the same day they tested HIV-positive. Subsequently they are offered starting ART the same day.
Measured outcomes in the CASCADE trial
Two primary outcomes will be measured to compare the care-cascade between the intervention and the standard of care arm. First, linkage to care within 3 months after having tested HIV positive (Definition: individual presents himself/herself at the HIV care clinic). Second, viral suppression 12 months after having tested HIV positive (viral load <100 copies/mL).
Recruitment for the CASCADE trial
From February 22 to July 17, 2016 teams consisting of lay counsellors and a study nurse visited villages and urban neighborhoods where they moved systematically from door-to-door proposing HIV testing to all household members present.
The first household visit took place on a working day, the second visit on the following week-end. The second visit aimed at proposing HIV-testing to those household members who were absent during the first visit. House-visits were recorded on tablets and synchronized with the visible impact server on daily basis.
To complete recruitment lay-counsellor teams had visited > 6000 households in 60 villages and > 20 urban neighborhoods in Butha-Buthe, proposing HIV testing to > 13'000 individuals. Recruitment was closed on July 17, 2016 when 274 individuals had been enrolled in the CASCADE-trial (137 in the standard of care and 137 in the intervention arm).
The following pictures provide some impressions from the home-based HIV testing campaigns
 One of the villages where door-to-door HIV testing was conducted
One of the villages where door-to-door HIV testing was conducted
Before HIV testing starts the team approaches the village chief to get permission to to home-based HIV testing in his village
Arrived at the village the team-leader organizes the team ensuring that each household will be visited.
And each lay-counsellor then starts walking to the households that were assigned to her/him
A lay-counsellor collects household-data before testing
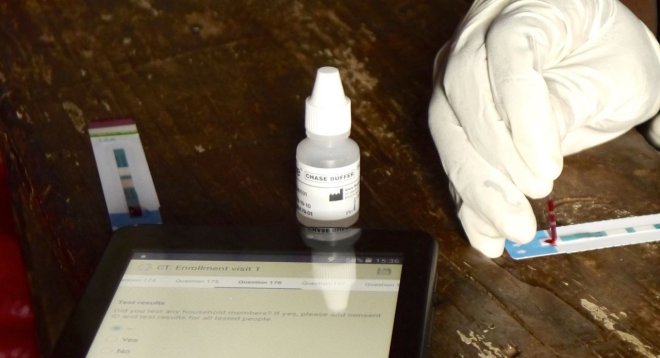 Tests are conducted in front of the individual, data are captured on tablets
Tests are conducted in front of the individual, data are captured on tablets
 One has to wait about 15 minutes for the test result to display on the test strip
One has to wait about 15 minutes for the test result to display on the test strip
If tested HIV positive, eligible and consenting individuals are enrolled in the CASCADE-trial

A remote village in the mountains near Rampai
The trial is described in more detail in the published study protocol:
http://bmcpublichealth.biomedcentral.com/articles/...
The trial is registered on:
https://www.clinicaltrials.gov/ct2/show/NCT0269202...
The Towards 90-90-90 Research Consortium
The CASCADE-trial project in Lesotho is conducted by a research consortium consisting of the Ministry of Health of Lesotho, the Swiss Tropical and Public Health Institute (www.swisstph.ch), SolidarMed (Swiss Organization for Health in Africa, www.solidarmed.ch) and Molecular Virology, Department of Biomedicine (DBM) of the University of Basel.
Contact: Niklaus Labhardt (Principal Investigator): n.labhardt(at)unibas.ch
On this website we provide updates on households covered by the home-based HIV testing campaign and number of individuals enrolled in the CASCADE-trial.
The project is supported through the following grants:
- r4d programme (www.r4d.ch), a joint funding initiative by the Swiss Agency for Development and Cooperation and the Swiss National Science Foundation (R4D Open Call IZ07Z0_160876/1)
- Stiftung für Infektiologie beider Basel
- Gottfried and Julia Bangerter-Rhyner Stiftung



Project Location
| Position | Location |
|---|---|
| Project location | Lesotho |
Project Team
-

Niklaus Labhardt
Sponsor-Investigator
